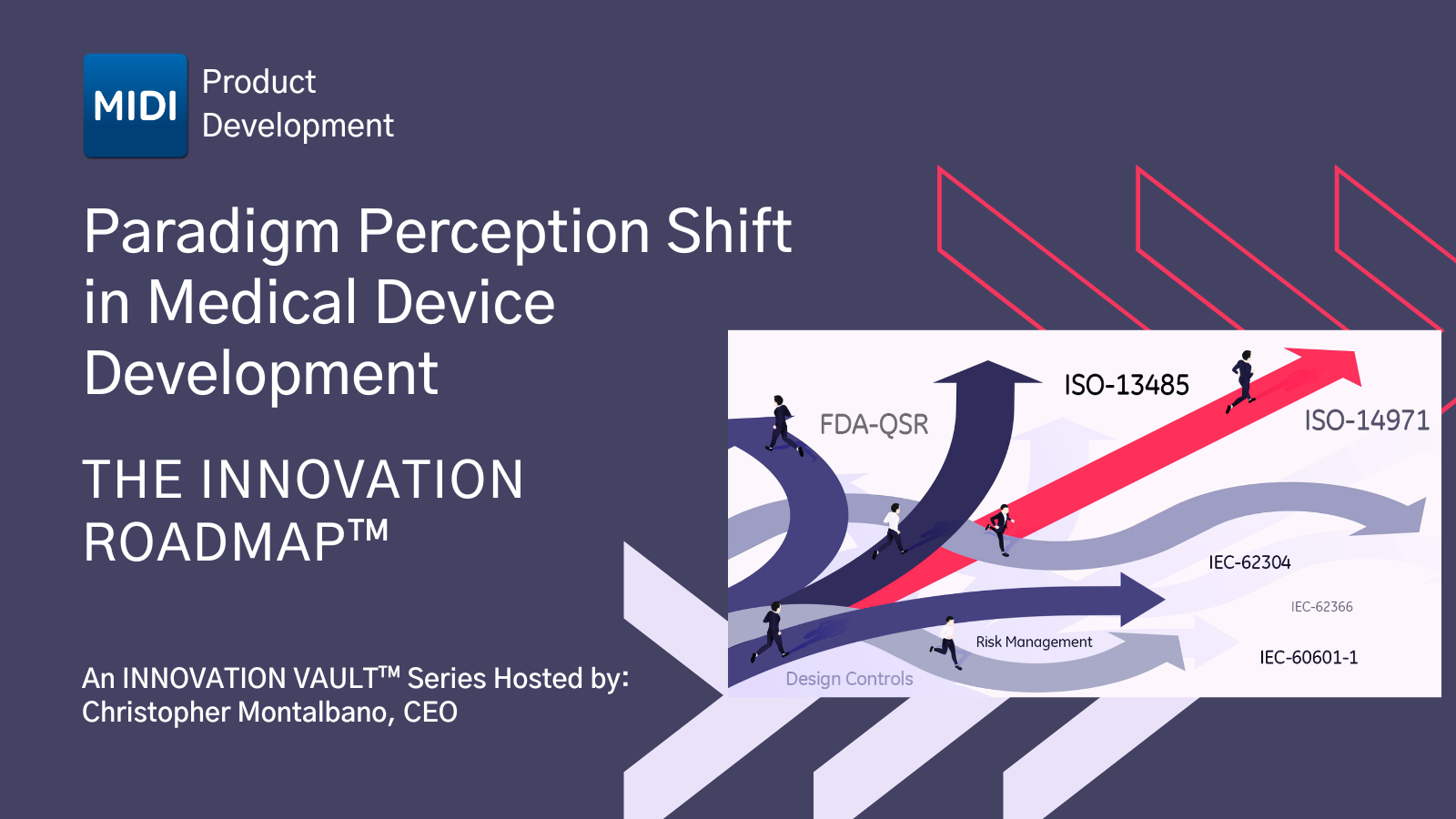
In the podcast series, PARADIGM PERCEPTION SHIFT in Medical Device Development: the INNOVATION ROADMAP™, we explore MIDI's DevelopmentDNA™ approach in which they infuse innovation and competitive differentiation into their client’s endeavors, supported by their team of engineers and usability experts tied to industrial designers. Utilizing this approach, their team evaluates and crafts responses to the functional, safety, business, and cost to manufacturer requirements of what MIDI refers to as “the golden standard approach,” which is partnered with rapid, AGILE development under the MIDI Quality 1st™ Umbrella. This series will illustrate this process, giving an inside look at the MIDI client experience tied to the utilization of FDA Guidance Regulation as well as Design Controls and Risk Management as a viable medical device development innovation platform.


